
Why Strategic Automation Is Now Essential in Medical Device Manufacturing
Medical device manufacturers are operating in an environment defined by complexity, competition, and rising expectations for quality and compliance. In this context, automation has shifted from a tactical improvement to a strategic necessity for organizations that need to scale reliably while protecting product quality and patient safety.
Automation as a Strategic Capability
Automation in medical device manufacturing should not be seen as a collection of standalone machines or isolated process upgrades. Instead, it functions best as part of an integrated operational strategy that improves consistency, supports compliance, enables scalable growth, and strengthens workforce capability. When approached deliberately, automation becomes a foundation for resilience, competitive advantage, and long term value.
Medical device production is unique in its combination of tight tolerances, patient safety expectations, and intense regulatory scrutiny. Products ranging from wearable diagnostics to implantable systems must deliver repeatable performance despite persistent labor shortages and the ongoing challenge of finding, training, and retaining skilled production talent.
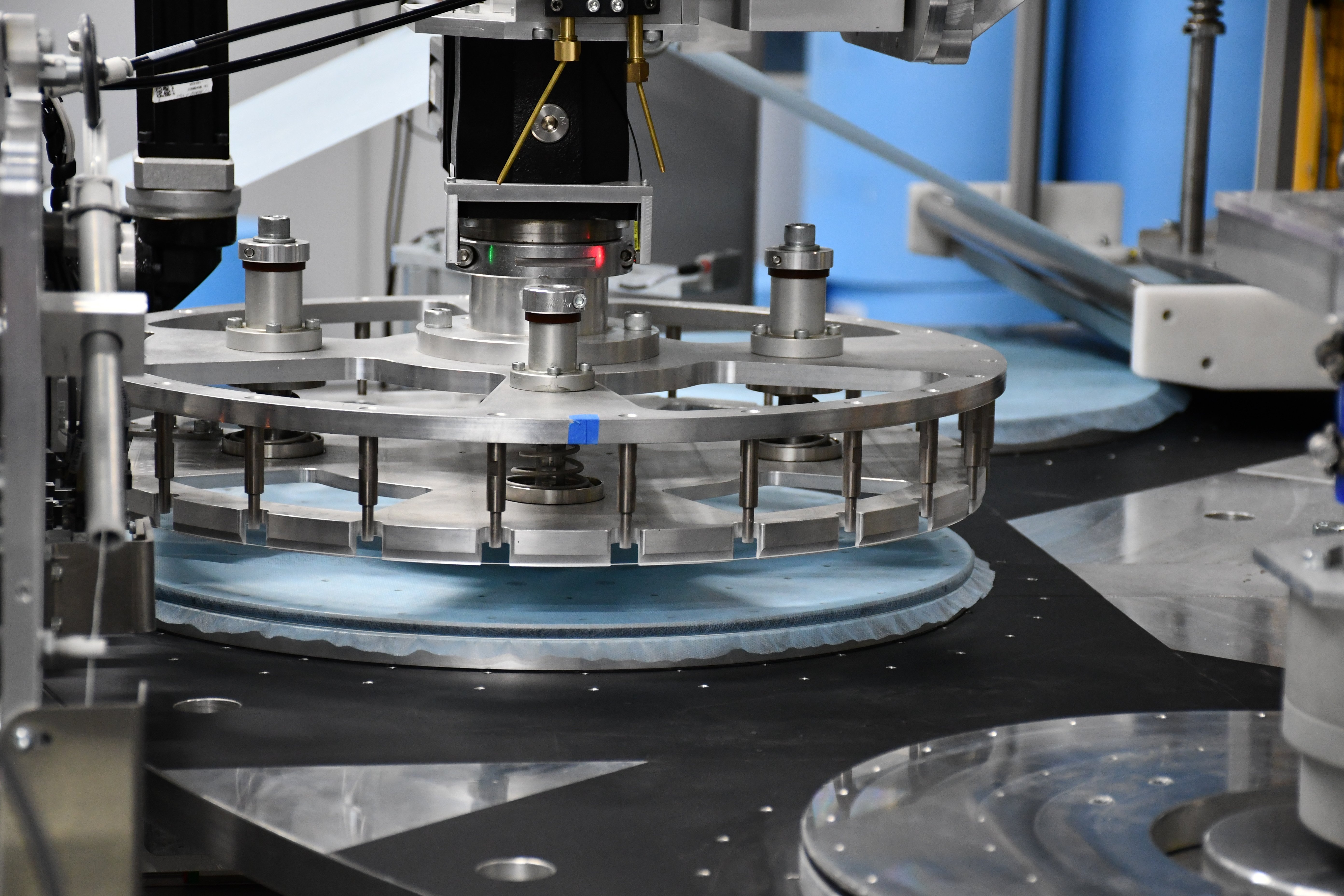
Addressing Variability and Operational Risk
Manual assembly and inspection introduce unavoidable variability driven by human factors such as fatigue, training differences, and turnover. Over time, this variability can affect product quality, extend release timelines, and increase operational risk. Automation addresses these challenges by standardizing process execution, embedding verification directly into production, and generating consistent data that supports both quality and compliance objectives.
At Integrion Automation, these challenges appear across programs from early pilot builds to high volume commercial production. Experience across multiple device categories reinforces the importance of automation strategies that balance precision, reliability, and scalability rather than focusing solely on speed.
Precision Through Consistency and In‑Process Verification
One of the most significant advantages of automation is repeatable precision. Automated systems execute assembly, handling, and inspection routines using the same parameters every cycle, which reduces defect rates and minimizes the need for rework or secondary inspection. Modern automation platforms also integrate sensing and inspection technologies directly into the production flow.
Vision inspection, functional testing, leak testing, and dimensional measurement can be performed at multiple stages during assembly. Capturing this data in real time allows manufacturers to identify process deviations early instead of discovering issues downstream, when they are more costly and disruptive. In regulated environments where quality issues can lead to compliance concerns or patient risk, this in process verification capability is especially valuable.
Traceability as a Built‑In Outcome
Traceability is a foundational requirement in medical device manufacturing, where regulators expect manufacturers to correlate materials, process parameters, and inspection results to each finished device or batch. Manual documentation systems increase the risk of transcription errors, inconsistent timestamps, and incomplete records that can complicate investigations and audits.
Automation inherently improves traceability by capturing data directly from equipment and sensors. Component lot information, process completion, inspection outcomes, and timestamps are automatically linked to serialized devices or batches, supporting faster batch release and strengthening confidence in data integrity. Automation providers such as Integrion Automation frequently collaborate with customer quality and regulatory teams early in system design so that traceability requirements are built into the core architecture rather than treated as an afterthought.
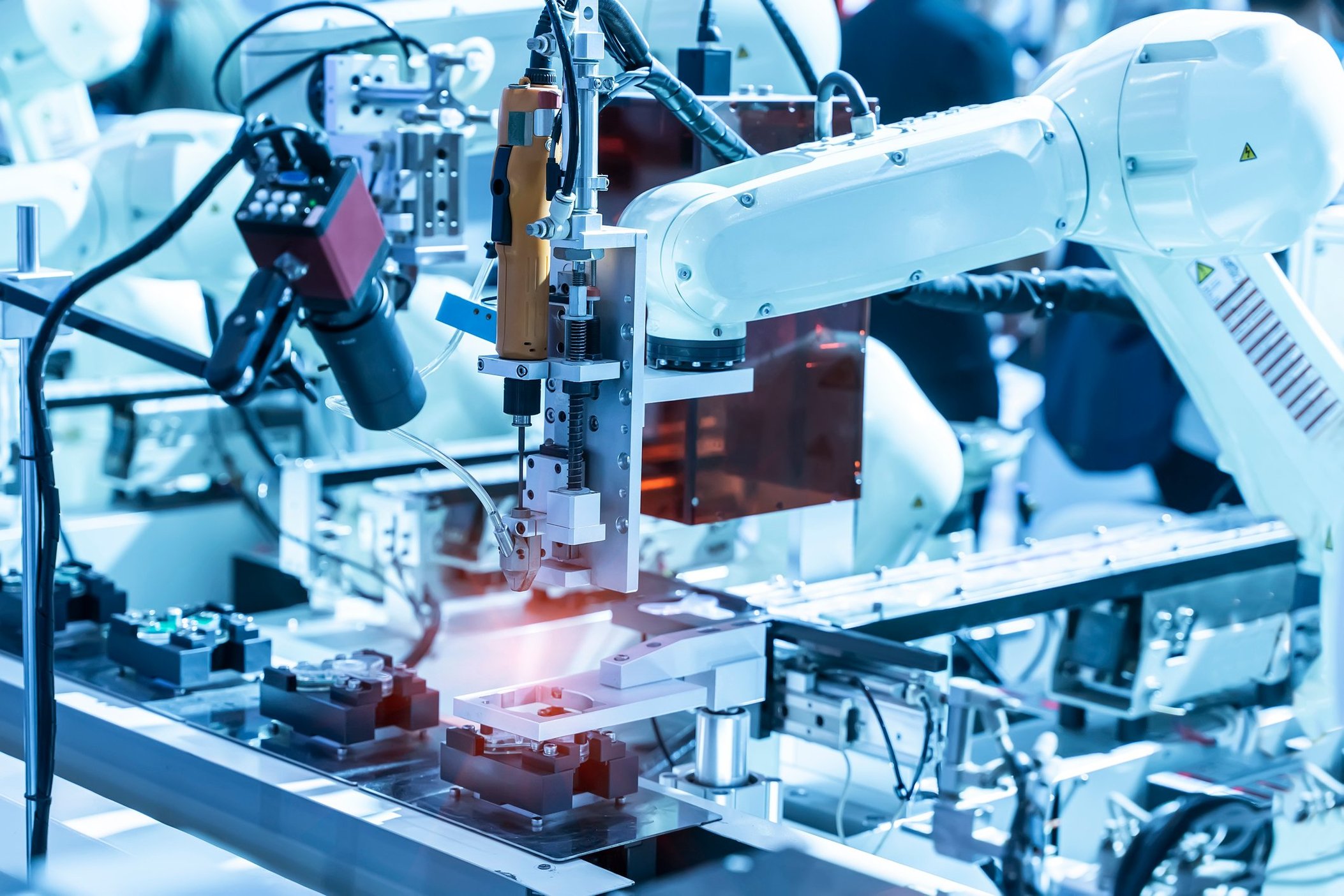
Designing Automation for Scalable Growth
A common misstep in adopting automation is focusing too aggressively on speed during initial deployment. While throughput matters, prioritizing extreme cycle times early can compromise yield, reliability, and overall equipment effectiveness, especially for complex new processes.
A more effective strategy is to begin with stable, well characterized processes operating at moderate rates. This allows teams to understand tolerance sensitivities, optimize tooling, and validate inspection methods before ramping up. Once performance is proven, capacity can increase through parallel equipment, targeted upgrades, or second generation system designs that build on this validated foundation.
This phased approach aligns well with the realities of medical device manufacturing, where products evolve and volume ramps occur over time. Modular automation systems, such as those Integrion Automation designs for life sciences applications, provide flexibility to adapt to new configurations without requiring complete system replacement.

